A 2.52 G Sample of a Compound
A 252- g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen to yield 423 g of CO2 and 101 g of H2O. The relative number of moles of C and S atoms in the compound can be obtained by converting grams to moles as shown.
Solved A 2 52 G Sample Of A Compound Containing Only Carbon Chegg Com
Another sample of the same compound of mass 414 g yields 211 g of SO3.
. Sample 3 contains 056238 g of nitrogen this is 993598 of the compound mass. Calculate the empirical formula of the compound. A third sample of mass 566g yielded 280 g of HNO3.
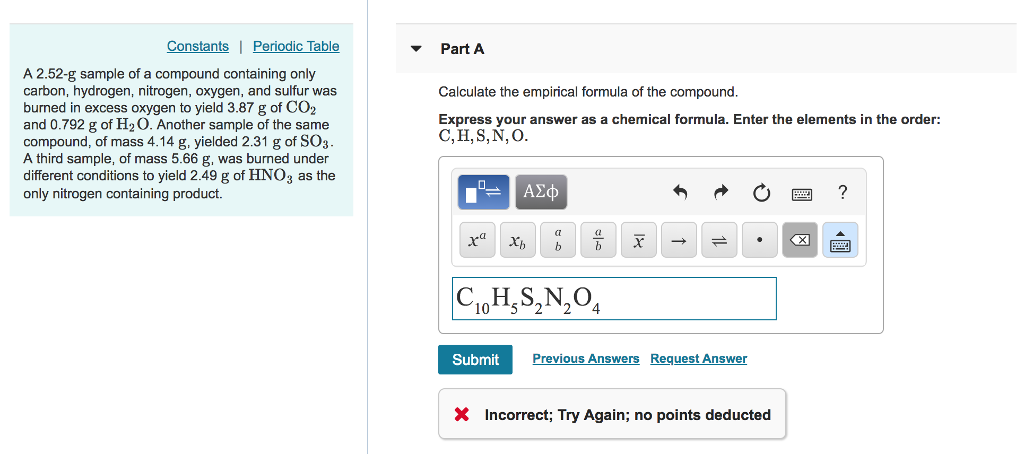
Another sample of the same compound of mass 414 g yields 211 g of SO3. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen to yield 387 g of CO2 and 0792 g of H20. A 252 g sample of a compound containing carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen gas to yield 436 grams of CO 2 and 0892 grams of H 2 O as the only carbon and hydrogen products respectively.
A third sample of mass 566 g was burned under different conditions to yield 227 g of HNO3 as the only nitrogen. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur is burned in excess oxygen to yield 423 g of CO2 and 101 g of H2O. Carbon 128544 g 0107022 moles.
Moles of N in 566 g of sample 004444 moles in 252 g of compound --- 004444 252 566 001979 moles mass of N --- 001979 x 14 02770 g All the nitrogen in the HNO 3 came from the 566 g sample. A 252 g sample of a compound containing carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen gas to yield 436 grams of CO 2 and 0892 grams of H 2 O as the only carbon and hydrogen products respectively. A 252 g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess O to yield 423 g of CO2 and 101 g H2O.
Another sample of the same compound of mass 414 g yielded 211 g of SO3. Another sample of the same compound of mass 414 g yielded 231 g of SO3 as the only sulfur containing product. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur is burned in excess oxygen to yield 423 g of CO2 and 101 g of H2O.
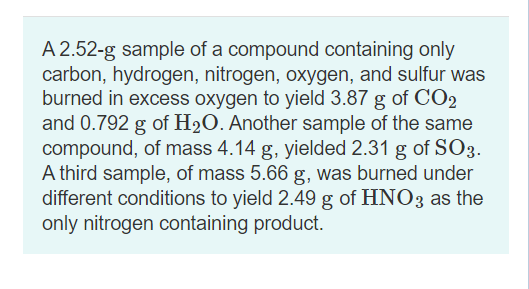
Calculate the empirical formula of the compound. Calculate the percent by mass of each element C mC mCxHy 100 748 g 1000 g 100 748 H mH mCxHy 100 252 g 1000 g 100 252. Another sample of the same compound of mass 414 g yielded 211 g of SO3.
Another sample of the same compound of mass 414 g yields 211 g of SO3. A 252 g sample of a compound containing carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen gas to yield 436 grams of Co and 0903 grams of H0 as the only carbon and hydrogen products respectively. Another sample of the same compound of mass 414 g yielded 175 g of SO3.
Anothers sample of the compound in compound of Mass. Another sample of the same compound of mass 414 g yielded 260 g of SO 3 as the only. The original sample weighed 252 g.
423 grams of two and 11 gram special. A third sample of mass 566 g yields 227 g of HNO3. Hydrogen 0125326 g 0124339 moles.
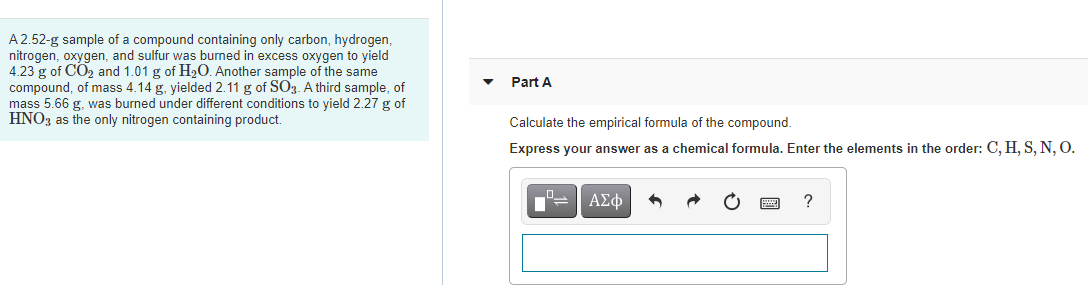
Calculate the empirical formula of the compound. A 252 gram sample of a compound containing only carbon hydrogen nitrogen option and sulfur has burned. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen to yield 423 g of CO2 and 101 g of H2O.
Hence 1000 g of the sample contains 158 g of C and 842 g of S. Another sample of the same compound of mass 414 g yielded 211 g of so3. A third sample of mass 566 g was burned under different conditions to yield 227 g of HNO3 as the only.
The mass of the compound is equal to the sum of the masses of the elements that form it. A second sample of the same compound of mass 414 g yielded 260 g of so as the only sulfur containing product. Calculate the empirical formula of the compound.
A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur is burned in excess oxygen to yield 423 g of CO2 and 101 g of H2O. Another sample of the same compound of mass 414 g yielded 235 g of SO3 as the only sulfur containing product. A third sample of mass 566 g yielded 227 g HNO3.
Notice the scaling from 566 g of compound to 252 g. Mass C H S N 11891 0100 06330 02770 21991. Another sample of the same compound of mass 414 g yielded 231 g of SO3.
A252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen to yield 423 g of co2 and 101 g of h2o. Determine the mass of the compound. Another sample of the same compound of mass 414g yielded g of SO3.
Calculate the empirical formula of the compound. Another sample of the same compound of mass 414 g yielded 211 g of SO3 as the only sulfur containing product. A third sample of mass 566 g was burned under different conditions to yield 227 g of hno3 as the only nitrogen.
Another sample of the same compound of mass 414 g yielded 211 g of SO3. Sample 2 contains 094108 g of sulfur this accounts of 094108414 100 22731 of the compound mass. A third sample of mass 566 g yields 227 g of HNO3.
MCxHy mC mH 748 g 252 g 1000 g. The mass of an element in a 1000-g sample of a compound is equal in grams to the percent of that element in the sample. A third sample of mass 566 g yields 227 g of HNO3.
A sample of a hydrocarbon is found to contain 748g carbon and 252g hydrogen. A reaction to determine the nitrogen content was carried out on a third sample with a mass of 566 g and yielded 188. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen to yield 423 g of CO2 and 101 g of H2O.
What is the empirical - 23508174. Another sample of the same compound of mass 414 g yielded 260 g of SO 3 as the only. A 252g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess O to yield 436g of CO2 and 0892g of H20.
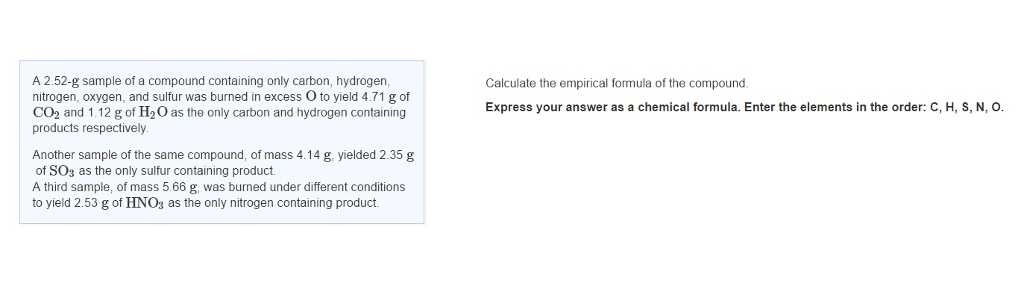
A third sample of mass 566 g was burned under different conditions to yield 249 g of HNO3 as the only nitrogen. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess O to yield 471 g of CO2 and 112 g of H2O as the only carbon and hydrogen containing products respectively. A 252 g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess O2 to yield 352 g of CO2 and 0839 g of H2O.
A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess O to yield 387 g of and 0792 g of H2o as the only carbon and hydrogen containing products respectively. Another sample of the same compound of mass 414 g yielded 211 g SO3. A 252-g sample of a compound containing only carbon hydrogen nitrogen oxygen and sulfur was burned in excess O to yield 423 g of CO2 and 101 g of H2O as the only carbon and hydrogen containing products respectively.
Solved A 2 52 G Sample Of A Compound Containing Only Carbon Chegg Com

Solved Constants Periodic Table Parta A 2 52 G Sample Of A Chegg Com
Solved A 2 52 G Sample Of A Compound Containing Only Carbon Chegg Com
0 Response to "A 2.52 G Sample of a Compound"
Post a Comment